How does α1Histidine102 affect the binding of modulators to α1β2γ2 GABAA receptors? molecular insights from in silico experiments†
Abstract
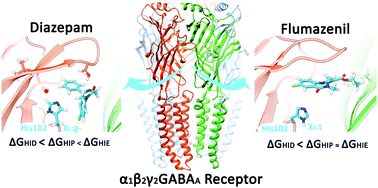
The activation of GABAA receptors by the neurotransmitter gamma-aminobutyric acid mediates the rapid inhibition response in the central nervous system of mammals. Many neurological and mental health disorders arise from alterations in the structure or function of these pentameric ion channels. GABAA receptors are targets for numerous drugs, including benzodiazepines, which bind to α1β2γ2 GABAA receptors with high affinity to a site in the extracellular domain, between subunits α1 and γ2. It has been established experimentally that the binding of these drugs depends on the presence of one particular amino acid in the α1 subunit: histidine 102. However, the specific role it plays in the intermolecular interaction has not been elucidated. In this work, we applied in silico methods to understand whether certain protonation and rotamer states of α1His102 facilitate the binding of modulators. We analysed diazepam binding, a benzodiazepine, and the antagonist flumazenil to the GABAA receptor using molecular dynamics simulations and adaptive biasing force simulations. The binding free energy follows changes in the protonation state for both ligands, and rotameric states of α1His102 were specific for the different compounds, suggesting distinct preferences for positive allosteric modulators and antagonists. Moreover, in the presence of diazepam and favoured by a neutral tautomer, we identified a water molecule that links loops A, B, and C and may be relevant to the modulation mechanism.


 Please wait while we load your content...
Please wait while we load your content...