Pressure induced topochemical polymerization of solid acrylamide facilitated by anisotropic response of the hydrogen bond network†
Abstract
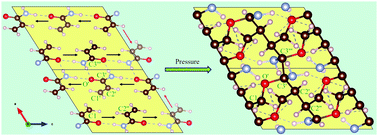
The pressure induced polymerization of molecular solids is an appealing route to obtain pure, crystalline polymers without the need for radical initiators. Here, we report a detailed density functional theory (DFT) study of the structural and chemical changes that occur in defect free solid acrylamide, a hydrogen bonded crystal, when it is subjected to hydrostatic pressures. While our calculations are able to reproduce experimentally measured pressure dependent spectroscopic features in the 0–20 GPa range, our atomistic analysis predicts polymerization in acrylamide at a pressure of ∼23 GPa at 0 K albeit through large enthalpy barriers. Interestingly, we find that the two-dimensional hydrogen bond network in acrylamide templates topochemical polymerization by aligning the atoms through an anisotropic response at low pressures. This results not only in conventional C–C, but also unusual C–O polymeric linkages, as well as a new hydrogen bonded framework, with both N–H⋯O and C–H⋯O bonds. Using a simple model for thermal effects, we also show that at 300 K, higher pressures significantly accelerate the transformation into polymers by lowering the barrier. Thus, application of pressure offers an alternative route for topochemical polymerization when higher temperatures are undesirable.


 Please wait while we load your content...
Please wait while we load your content...