Threshold photoionization shows no sign of nitryl hydride in methane oxidation with nitric oxide†
Abstract
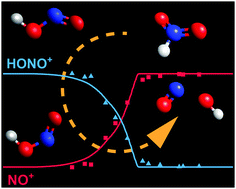
Methane was doped with nitric oxide and oxidized in a high-pressure flow reactor. The nitrogen chemistry during partial oxidation was studied using photoelectron photoion coincidence spectroscopy with vacuum ultraviolet synchrotron radiation. The adiabatic ionization energy of nitrous acid, HONO, has been determined as 10.95 ± 0.03 eV. The HONO breakdown diagram was plotted based solely on the measured parent signal and the computed Franck–Condon envelope of trans-HONO, confirming the trans-HONO dissociative photoionization threshold to NO+ + ˙OH at 11.34 eV. The spectra show strong indication for the presence of cis-HONO. We expected the m/z 47 photoion mass selected threshold photoelectron signal to rebound near 12 eV, i.e., at the ionization energy of nitryl hydride, the third HNO2 isomer. Recent computational studies suggest nitryl hydride is formed at a rate similar to trans-HONO, is more thermally stable than nitrous acid, its cation is bound, and its photoelectron spectrum is predicted to exhibit a strong origin band near 12 eV. The absence of its mass selected threshold photoelectron signal shows that nitryl hydride is either not formed in measurable amounts or is consumed faster than nitrous acid, for instance by isomerization to trans-HONO.


 Please wait while we load your content...
Please wait while we load your content...