Tracer diffusion coefficients of Li+ ions in c-axis oriented LixCoO2 thin films measured by secondary ion mass spectrometry
Abstract
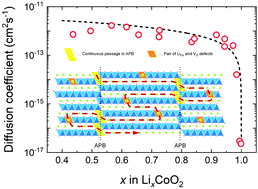
Lithium diffusion is a key factor in determining the charge/discharge rate of Li-ion batteries. Herein, we study the tracer diffusion coefficient (D*) of lithium ions in the c-axis oriented LiCoO2 thin film using secondary ion mass spectrometry (SIMS). We applied a step-isotope-exchange method to determine D* in the Li-extracted LixCoO2. The observed values of D* ranged from 2 × 10−12 to 3 × 10−17 cm2 s−1 depending on the compositions in the range of 0.4 < x < 1.0. Approaching the stoichiometric composition (x = 1.0), D* decreases steeply to the minimum, which can be explained by the vacancy diffusion mechanism. Electrochemically determined diffusion coefficients corrected by thermodynamic factors are found to be in good agreement with D* determined by our method, over a wide range of compositions. The c-axis diffusion was explained by the migration of Li+ ions from one layer to another through additional diffusion channels, such as antiphase boundaries and a pair of Li antisite and oxygen vacancies in cobalt oxide layers.


 Please wait while we load your content...
Please wait while we load your content...