Tuning the transition barrier of H2 dissociation in the hydrogenation of CO2 to formic acid on Ti-doped Sn2O4 clusters†
Abstract
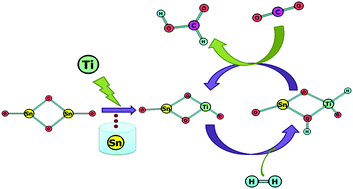
A density functional theory study has been performed to investigate cation-doped Sn2O4 clusters for selective catalytic reduction of CO2. We study the influence of Si and Ti dopants on the height of the H2 dissociation barrier for the doped systems, and then the subsequent mechanism for the conversion of CO2 into formic acid (FA) via a hydride pinning pathway. The lowest barrier height for H2 dissociation is observed across the ‘Ti–O’ bond of the Ti-doped Sn2O4 cluster, with a negatively charged hydride (Ti–H) formed during the heterolytic H2 dissociation, bringing selectivity towards the desired FA product. The formation of a formate intermediate is identified as the rate-determining step (RDS) for the whole pathway, but the barrier height is substantially reduced for the Ti-doped system when compared to the same steps on the undoped Sn2O4 cluster. The free energy of formate formation in the RDS is calculated to be negative, which reveals that the hydride transfer would occur spontaneously. Overall, our results show that the small-sized Ti-doped Sn2O4 clusters exhibit better catalytic activity than undoped clusters in the important process of reducing CO2 to FA when proceeding via the hydride pinning pathway.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...