The role of solute polarity on methanol–silica interfacial solvation: a molecular dynamics study†
Abstract
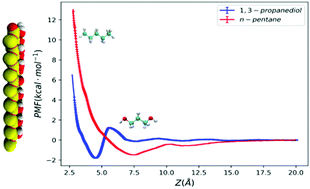
The solvation structure and dynamics of small organic molecules at the methanol–silica interface are important for understanding the reaction dynamics in heterogeneous catalysis as well as the transport mechanisms in liquid chromatography. The role of solute polarity in interfacial solvation at the methanol–silica interface has been investigated via umbrella sampling molecular dynamics (MD) simulations and 1,3-propanediol and n-pentane were selected as representative species of polar and apolar solutes. Free energy calculations reveal that it took a similar free energy cost to transfer both solute molecules from the interface to the bulk, despite the huge difference in their polarities. The 1,3-propendiol molecule can penetrate the adsorbed methanol layer and form hydrogen bonds with the silica surface with its backbone perpendicular to the silica surface, resulting in a significant slowdown of translational and rotational dynamics. Further analysis of solvent density and solute orientations suggest that at the minimum of the adsorption free energy curve, the 1,3-propanediol molecule is in a desolvated state, while n-pentane is in a solvated state. The collective effect of solute concentration has also been studied by unbiased MD simulations, and the free energy barriers of transferring the solute molecule from the interface to bulk, as well as the parallel diffusion coefficients at the interface, show a non-monotonic dependence on solute concentration, which can be related to the crowded environment in the interfacial layers.


 Please wait while we load your content...
Please wait while we load your content...