Effect of an external electric field, aqueous solution and specific adsorption on segregation of PtML/MML/Pt(111) (M = Cu, Pd, Au): a DFT study†
Abstract
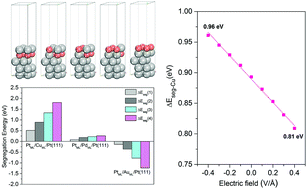
The oxygen reduction reaction (ORR) that occurs on the outermost layer of electrocatalysts is significantly affected by the composition and structure of the electrocatalysts. During the preparation of PtM alloy electrocatalysts, high-temperature annealing in an inert or reducing atmosphere could promote the segregation of M toward the core, forming a highly active Pt-skin structure. However, under fuel cell operating conditions, the adsorption of oxygen-containing groups could stimulate the easily dissolved M to segregate to the surface, reducing the activity and stability of the electrocatalysts. In this work, we conducted segregation energy calculation of PtM (M = Cu, Pd, Au) electrocatalysts under specific adsorption (SA), aqueous solution (AS) and an external electric field (EEF) with a density functional theory method. It was found that different factors have different effects on the segregation energy: ΔΔESA ≫ ΔΔEEEF > ΔΔEAS. The coupling effects have also been considered and compared: ΔΔESA+EEF > ΔΔESA+AS > ΔΔEEEF+AS. When including all three factors, the change of segregation energy 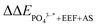 could reach 1.63 eV. Therefore, operating conditions have a noteworthy influence on the segregation behavior of PtM ORR electrocatalysts, which should be considered in the further design of PtM ORR electrocatalysts.
could reach 1.63 eV. Therefore, operating conditions have a noteworthy influence on the segregation behavior of PtM ORR electrocatalysts, which should be considered in the further design of PtM ORR electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...