A theoretical insight into the formation of chalcogen bonding (ChB) interactions involving coordinated DMSO molecules as σ-hole donors and benzoate groups as σ-hole acceptors in a dinuclear copper(ii) complex†
Abstract
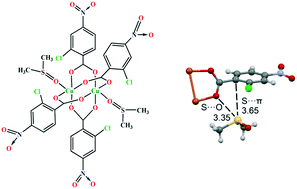
A dimeric copper(II) complex, [Cu2(μ1,3-L)4(DMSO)2] (HL = 2-chloro-4-nitrobenzoic acid), was synthesized and characterized by standard spectroscopic methods and X-ray analysis. In the solid state, conventional C–H⋯O and π–π stacking interactions are important forces governing the X-ray packing, as evidenced by Hirshfeld surface analysis. The formation of two chalcogen bonding (ChB) interactions involving coordinated DMSO molecules as σ-hole donors and the O atoms of carboxylate groups as acceptors has been also described and studied by means of DFT calculations. The effect of the Cu coordination of DMSO on the intensity of the sigma holes has been studied using MEP surface analysis. The S⋯O and S⋯π contacts have been characterized by using a combination of QTAIM and NCI plot computational tools.
- This article is part of the themed collection: Supramolecular & Polymorphism


 Please wait while we load your content...
Please wait while we load your content...