2D and 3D silver-based coordination polymers with thiomorpholine-4-carbonitrile and piperazine-1,4-dicarbonitrile: structure, intermolecular interactions, photocatalysis, and thermal behavior†
Abstract
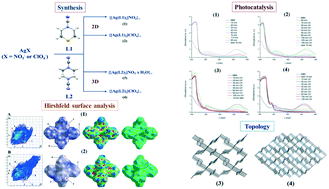
Four silver-based coordination polymers, {[Ag(L1)2]NO3}∞ (1), {[Ag(L1)2]ClO4}∞ (2), {[Ag(L2)2]NO3·H2O}∞ (3) and {[Ag(L2)2]ClO4}∞ (4), were synthesized using the thiomorpholine-4-carbonitrile (L1) and piperazine-1,4-dicarbonitrile (L2) ligands. Compounds 1 and 2 are two-dimensional, while 3 and 4 are three-dimensional. L1 and L2 are 1,4-bis-monodentate ligands in all compounds, while Ag(I) ions are four-coordinated in a slightly distorted tetrahedral geometry. Topological analysis in standard representations suggests that underlying nets in 1 and 2 have an sql topology, while 3 and 4 exhibit a dia topology. Thermal analysis shows that 3 loses crystalline water at room temperature, while other compounds show good thermal stability. All compounds show good photocatalytic activity for photocatalytic degradation of the mordant blue 9 dye, with reaction rates in the range 0.029 to 0.061 min−1. The best result was obtained for compound 4, which can be correlated to its largest lattice volume.
- This article is part of the themed collection: Coordination Networks


 Please wait while we load your content...
Please wait while we load your content...