Construction of three new Co(ii)-organic frameworks based on diverse metal clusters: highly selective C2H2 and CO2 capture and magnetic properties†
Abstract
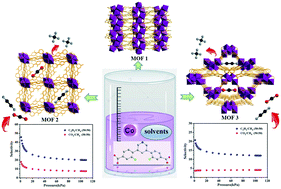
Using a symmetrical aromatic carboxylic acid ligand, 4,4′-(pyridine-3,5-diyl)bis(3-fluorobenzoic acid) (H2L), three Co(II)-based metal–organic frameworks, namely, {[Co3(L)2(H2O)2(HCOO)2]}n (1), {[Co3(L)3(H2O)]·4H2O·2DMF}n (2) and {[Co5(L)4(H2O)4(μ3-H2O)2]·11H2O·6DMF·2(NO3)}n (3), have been solvothermally synthesized. Structural analysis demonstrates that 1–3 possess diverse secondary structural units (SBUs). 1 is a 3D network with a binodal (3,6)-connected Schläfli topology based on trinuclear [Co3(COO)4(HCOO)2N2] SBUs. Compound 2 possesses different trinuclear [Co3(COO)6N3(μ3-H2O)] SBUs, which are further extended by L2− to produce a 3D porous network containing 1D channels with a triangular shape along the c axes, and with an uncommon (3,9)-connected topology. In particular, 3 is constructed from unique pentanuclear [Co5(μ3-H2O)2N4(COO)8]2+ SBUs to form a (3,12)-connected network, and contains one-dimensional triangular channels. Furthermore, the desolvated frameworks of 2 and 3 show selective adsorption for C2H2 and CO2 over CH4 at 273 and 298 K. Moreover, the magnetic properties of 1–3 have been completely examined.
- This article is part of the themed collection: Coordination Networks


 Please wait while we load your content...
Please wait while we load your content...