Design, synthesis, and characterization of pyridine-containing organic crystals with different substitution positions using solvothermal method†
Abstract
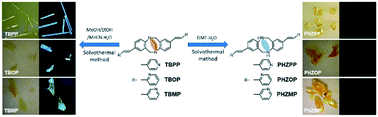
The organic crystals of Tröger's base (TB) analogs with ortho-/meta-substituted terminal pyridine units, 2,8-(6H,12H-5,11-methanodibenzo[b,f]diazocineylene)-di(o-ethenylpyridine) (TBOP) and 2,8-(6H,12H-5,11-methanodibenzo[b,f]diazocineylene)-di(m-ethenylpyridine) (TBMP), were prepared using solvothermal method. By alternating the solution system and ratio, not only the TBOP and TBMP crystals but also the central-methylene-removed 5,6,11,12-tetrahydro-2,8-dimethylphenhomazine-di(o-ethenyl-N-pyridine) (PHZOP) and 5,6,11,12-tetrahydro-2,8-dimethylphenhomazine-di(m-ethenyl-N-pyridine) (PHZMP) crystals could be obtained from TBOP and TBMP. Furthermore, single-crystal XRD, 1H NMR, 13C NMR, fluorescence spectrometry, combined with Hirshfeld surface calculation, were performed to analyze the relationship between crystal structures and photophysical properties. Since the pyridine unit can only act as a hydrogen bond acceptor in the crystal packing process, the competition and the synergic effects between the pyridine H-acceptor and other intermolecular interaction sites caused by the small alteration of N-substitution lead to significant changes in crystal structures and photophysical properties.
- This article is part of the themed collection: Crystal Growth


 Please wait while we load your content...
Please wait while we load your content...