Ligand field and anion-driven structures and magnetic properties of dysprosium complexes†
Abstract
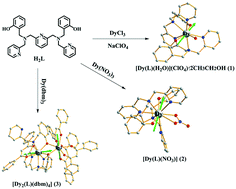
Based on the organic ligand 2,2′-(((pyridine-2,6-diylbis(methylene))bis((pyridin-2-ylmethyl)-azanediyl))-bis(methylene))diphenol (H2L), and dysprosium salts with different anions, two mononuclear and one dinuclear complexes, namely, [Dy(L)(H2O)](ClO4)·2CH3CH2OH (1), [Dy(L)(NO3)] (2) and [Dy2(L)(dbm)4] (3, Hdbm = dibenzoylmethane), were synthesized and structurally and magnetically characterized. In complexes 1 and 3, the Dy centers are octa-coordinated with triangular dodecahedral (local D2d symmetry) environments. Complex 1 is dimeric by hydrogen bond interactions between coordinated water molecules. In complex 3, two DyIII ions are linked by two phenoxy oxygen atoms to form a dinuclear cluster. The DyIII ion in complex 2 is nine-coordinated and adopts a spherical capped square antiprism geometry with a C4v symmetry. Magnetic measurements and theoretical calculations reveal that the magnetic couplings between two phenoxyl-bridging DyIII ions in complex 3 are antiferromagnetic and mostly originate from the exchange coupling. Both complexes 1 and 2 exhibit single molecule magnet behaviors under zero direct-current field with effective energy barriers of 50.94 K and 12.26 K, respectively. Complex 3 only exhibits frequency-/temperature-dependent out-of-phase signals, and no peak is observed.
- This article is part of the themed collection: Crystal Growth


 Please wait while we load your content...
Please wait while we load your content...