Effect of modification of binary solvent molecules on ε-CL-20 crystal morphology: a molecular dynamics study†
Abstract
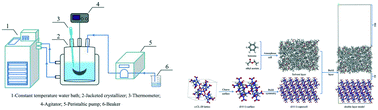
In this work, the effect of 13 binary solvent systems on ε-CL-20 crystal growth was studied by a combined experimental and simulative method. The solvent–crystal interactions were considered by using the modified attachment energy (MAE) model and then the modified ε-CL-20 crystal morphologies in the different binary solvent systems were predicted by molecular dynamics simulation. The contrastive crystals in the different systems were prepared by a solvent–antisolvent crystallization method. The results demonstrate that the growth morphology derived from the MAE model is in good accordance with that observed in the experiments. The solvent–crystal interactions are mainly composed of hydrogen bonding and Coulomb interactions, and the surface roughness is found to be important to the solvent molecule adsorption. Moreover, due to the steric hindrance and polarity of the antisolvents, the diffusion coefficient of the solvent molecules is not positively correlated with the interaction energy.
- This article is part of the themed collection: Crystal Growth


 Please wait while we load your content...
Please wait while we load your content...