Efficient removal of Pb2+ and Cd2+ using a Cu(i)–Br coordination polymer constructed with an amino-rich ligand†
Abstract
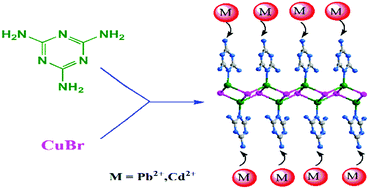
In this work, a Cu(I)–Br coordination polymer Cu2Br2(MA)2 (1) (MA = melamine) with an amino-rich ligand was synthesized and applied for the removal of Pb2+ and Cd2+ successfully. The effect of various parameters like pH, initial metal ion concentration, and contact time on the adsorption performance of 1 was investigated. The adsorption of Pb2+ and Cd2+ were in good agreement with the pseudo-second-order kinetic model and Langmuir isotherm model. The maximum adsorption capacities for Pb2+ and Cd2+ were 598.8 mg g−1 and 2175 mg g−1 at 298.15 K, respectively. The high adsorption capacities were attributed to the coordination interaction between the amino groups of melamine molecules and Pb2+ and Cd2+ ions. Moreover, the adsorption performance of Pb2+ and Cd2+ ions showed no obvious decrease even after six cycles.
- This article is part of the themed collection: Coordination Networks


 Please wait while we load your content...
Please wait while we load your content...