Proton sponge lead halides containing 1D polyoctahedral chains†
Abstract
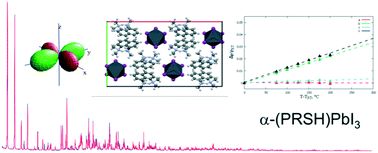
Hybrid one-dimensional lead halides, containing the protonated 1,8-bis(dimethylamino)naphthalene moiety (C14H19N2, monoprotonated “proton sponge”), were prepared by simple one-pot methods and investigated in terms of crystal structure, morphology, thermal stability and electronic properties. The as-precipitated (C14H19N2)PbBr3 and (C14H19N2)PbI3 species are isostructural and crystallize in the orthorhombic Pbca space group, resulting in 1D crystal phases with ([PbX3]−)∞ chains (built by face-sharing [PbX6] octahedra; X = Br, I), among which the (C14H19N2)+ cations are inserted. The two compounds display complete miscibility in the solid state: both (C14H19N2)PbI2Br and (C14H19N2)PbIBr2 crystal phases were successfully synthetized and do not show segregation effects. TGA and DSC measurements showed that, while all these materials are stable up to 250 °C, the iodine-rich (C14H19N2)PbI3 and (C14H19N2)PbI2Br species undergo an irreversible phase transition around 220 °C to a high-temperature phase, with a smaller molar volume and an apparent symmetry lowering (to orthorhombic Pca21). In these two cases, variable-temperature X-ray diffraction measurements showed a substantial crystal-to-crystal transformation, without intermediate melting or amorphization. The thermal expansion coefficients of (C14H19N2)PbI3 and (C14H19N2)PbBr3 are highly anisotropic and on the order of 10−4 K−1 along the b crystallographic axis. Microscopic and spectroscopic measurements were performed to complement the structural analysis of the samples: band gaps in the near UV range (3.3 and 3.8 eV for (C14H19N2)PbI3 and (C14H19N2)PbBr3, respectively) were obtained.
- This article is part of the themed collection: Coordination Networks


 Please wait while we load your content...
Please wait while we load your content...