Hydrates of adenosine 3′,5′-cyclic monophosphate sodium and their transformation†
Abstract
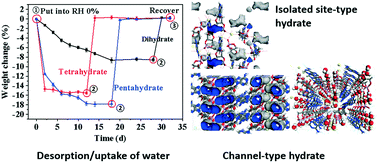
Almost all salt forms of nucleotides exist as crystalline hydrates. In this study, the hydrates of adenosine-3′,5′-cyclic phosphate sodium (cAMPNa·nH2O, n = 2, 4, 5, including its methanol solvate of n = 3) were systematically investigated for the crystal structures, their humidity sensibility, the transformation behaviors mediated by humidity, and the relationship between crystal water behaviors and specific structures. It was found that there are some major differences in crystal morphologies, molecular conformation, bonding profiles, and crystal water distribution in the lattices of different hydrates. The difference in the desorption dynamics of crystal water for the different hydrates was mainly attributed to their steric effects in the crystal lattice despite the relation to the interaction of water molecules with sodium ions and cAMP anions. The reversible conversions between the hydrates and their corresponding low hydrates or even anhydrates occur along with desorption/uptake of crystal water mediated by humidity, yet the reversible transformation capability would be disabled once the host structures were destroyed due to excessive loss of crystal water.
- This article is part of the themed collection: Supramolecular & Polymorphism


 Please wait while we load your content...
Please wait while we load your content...