Copper(i)-catalyzed tandem synthesis of 2-acylquinolines from 2-ethynylanilines and glyoxals†
Abstract
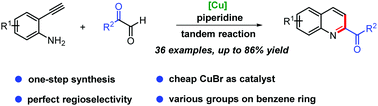
An efficient one-step synthesis of 2-acylquinolines using a copper-catalyzed tandem reaction of 2-ethynylanilines with glyoxals in the presence of piperidine has been developed. This new protocol successfully avoids multi-step operation and the use of highly toxic cyanides required in traditional methods, and provides a practical tool for synthetic and pharmaceutical chemists. Various 2-acylquinolines are obtained with perfect regioselectivity in moderate to good yields (up to 86%). The potential synthetic utility of this method is exemplified by a large-scale experiment and synthetic transformation of the products.


 Please wait while we load your content...
Please wait while we load your content...