Haloarene-guided cascade arylation of cyclic vinylogous esters under palladium catalysis†
Abstract
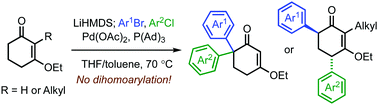
A method is presented for the synthesis of diaryl cyclic vinylogous esters. The sequence of C(sp3)–H arylation events is programed under the differentiated reactivity of the aryl halides, and the optimized reaction system is effectively diverted from producing dihomo-arylated products. The site selectivity of the second arylation is notably modulated by the substitution pattern of the substrates.


 Please wait while we load your content...
Please wait while we load your content...