Stereoselective total synthesis of (±)-vindeburnol and (±)-16-epi-vindeburnol†
Abstract
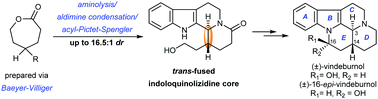
A concise stereoselective total synthesis of (±)-vindeburnol and its epimer (±)-16-epi-vindeburnol is presented. This synthetic work features the utilization of Baeyer–Villiger oxidation to install different types of lactone substrate, and a sequence of aminolysis, aldimine condensation and acyl-Pictet–Spengler to deliver the crucial trans-fused indoloquinolizidine scaffold with high-level diastereocontrol.


 Please wait while we load your content...
Please wait while we load your content...