Preparation of selenyl 1,3-oxazines via PhICl2/Cu2O-promoted aminoselenation of O-homoallyl benzimidates with diselenides†
Abstract
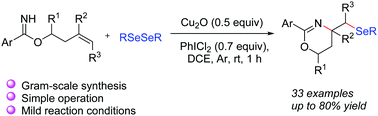
A practical electrophilic aminoselenation of O-homoallyl benzimidate with diselenides promoted by PhICl2/Cu2O has been developed. The easily available and stable diselenides were used as selenium sources. Various selenyl 1,3-oxazines, which are important frameworks in medicinal and biological chemistry, were easily obtained in moderate to good yields for the first time. Easy scaleup and scalability make this method attractive for the preparation of other valuable organoselenides.


 Please wait while we load your content...
Please wait while we load your content...