Synthesis and evaluation of peptidic thrombin inhibitors bearing acid-stable sulfotyrosine analogues†
Abstract
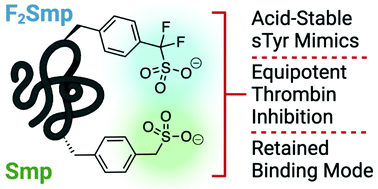
Tyrosine sulfation is an important post-translational modification of peptides and proteins which underpins and modulates many protein–protein interactions. In order to overcome the inherent instability of the native modification, we report the synthesis of two sulfonate analogues and their incorporation into two thrombin-inhibiting sulfopeptides. The effective mimicry of these sulfonate analogues for native sulfotyrosine was validated in the context of their thrombin inhibitory activity and binding mode, as determined by X-ray crystallography.


 Please wait while we load your content...
Please wait while we load your content...