Fragment evolution for GPCRs: the role of secondary binding sites in optimization†
Abstract
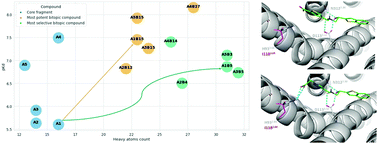
We developed a docking-based fragment evolution approach that extends orthosteric fragments towards a less conserved secondary binding pocket of GPCRs. Evaluating 13 000 extensions for the β1- and β2-adrenergic receptors we synthesized and tested 112 bitopic molecules. Our results confirmed the positive contribution of the secondary binding pocket to both potency and selectivity optimizations.


 Please wait while we load your content...
Please wait while we load your content...