2,2′-Bipyridine-α,α′-trifluoromethyl-diol ligand: synthesis and application in the asymmetric Et2Zn alkylation of aldehydes†‡
Abstract
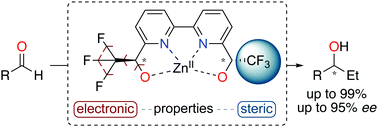
A chiral 2,2′-bipyridine ligand (1) bearing α,α′-trifluoromethyl-alcohols at 6,6′-positions was designed in five steps affording either the R,R or S,S enantiomer with excellent stereoselectivities, i.e. 97% de, >99% ee and >99.5% de, >99.5% ee, respectively. The key step for reaching high levels of stereoselectivity was demonstrated to be the resolution of the α-CF3-alcohol using (S)-ibuprofen as the resolving agent. An initial application for the 2,2′-bipyridine-α,α′-CF3-diol ligand was highlighted in the ZnII-catalyzed asymmetric ethylation reaction of aromatic, heteroaromatic, and aliphatic aldehydes. Synergistic electron deficiency and steric hindrance properties of the newly developed ligand afforded the corresponding alcohols in good to excellent yields (up to 99%) and enantioselectivities (up to 95% ee). As observed from single crystal diffraction analysis, the complexation of the 2,2′-bipyridine-α,α′-CF3-diol ligand generates an unusual hexacoordinated ZnII.


 Please wait while we load your content...
Please wait while we load your content...