nN → π*Ar interactions stabilize the E-ac isomers of arylhydrazides and facilitate their SNAr autocyclizations†
Abstract
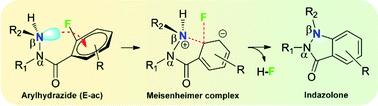
We describe a novel mechanism of stabilization of the E-ac isomer of an arylhydrazide via nN → π*Ar interactions. We further show that when a leaving group (F) is present at the ortho-position of the carbonyl group of such an arylhydrazide, the nN → π*Ar interaction facilitates an SNAr autocyclization reaction to produce indazolone, an important heterocycle with biological activity. Faster autocyclization of arylhydrazide is observed when an electron withdrawing group is present in the aryl ring, which is a characteristic of SNAr reactions.


 Please wait while we load your content...
Please wait while we load your content...