Cu-catalyzed C(sp2)–N-bond coupling of boronic acids and cyclic imides†
Abstract
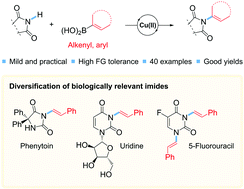
A general Cu-catalyzed strategy for coupling cyclic imides and alkenylboronic acids by forming C(sp2)–N-bonds is reported. The method enables the practical and mild preparation of (E)-enimides. A large range of cyclic imides are allowed, and di- and tri-substituted alkenylboronic acids can be used. Full retention was observed in the configuration of the alkene double bond in the coupled products. The method is also applicable for preparing N-arylimides, using arylboronic acids as coupling partners. The usefulness of this strategy is exemplified by the convenient derivatization of the chemotherapy medication 5-flurouracil, the nucleoside uridine and the anti-epileptic drug phenytoin.


 Please wait while we load your content...
Please wait while we load your content...