Enantioselective total synthesis of (+)-rubrobramide, (+)-talaramide A, and (−)-berkeleyamide D by a skeletal diversification strategy†
Abstract
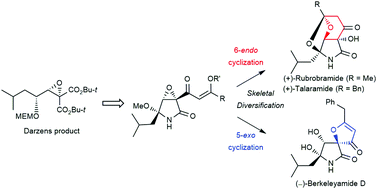
A unified synthesis of (+)-rubrobramide, (+)-talaramide A, and (−)-berkeleyamide D was achieved from the vinylogous esters by a skeletal diversification strategy based on regioselective 5-exo or 6-endo cyclization. This report describes the first enantioselective total synthesis of (+)-rubrobramide and (+)-talaramide A. Additionally, synthetic spirocyclic lactam compounds, including (−)-berkeleyamide D, showed moderate inhibitory activity against amyloid-β aggregation for the potential treatment of Alzheimer's disease.


 Please wait while we load your content...
Please wait while we load your content...