Recent advances in radical-based C–F bond activation of polyfluoroarenes and gem-difluoroalkenes
Abstract
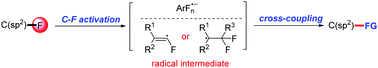
The direct employment of polyfluoroarenes and gem-difluoroalkenes as building blocks is regarded as one of the most effective and straightforward strategies for the introduction of fluorine-containing moieties into organic skeletons. Accordingly, radical chemistry has gradually become a mild and powerful method for the activation of their C–F bonds. The radical-based transformations of polyfluoroarenes and gem-difluoroalkenes can be primarily categorized into three types based on the specific intermediates involved: (1) multifluoroaryl radical anions, (2) monofluoroalkenyl radicals and (3) other radicals. Compared with the more established multifluoroaryl radical anion pathway, the monofluoroalkenyl radical-involved cross-coupling reaction can proceed through C-radical cross-coupling, radical addition/elimination or the hydrogen atom transfer process. For the presented examples in this review, the typical reaction modes, substrate scope, radical-involved mechanisms, and late-stage applications in the modification of bioactive molecules are discussed, aiming to provide a comprehensive overview of the recent advances of the radical-based transformations of polyfluoroarenes and gem-difluoroalkenes.


 Please wait while we load your content...
Please wait while we load your content...