Mass spectrometry enables the discovery of inhibitors of an LPS transport assembly via disruption of protein–protein interactions†
Abstract
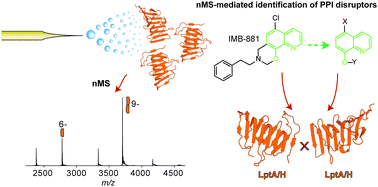
We developed a native mass spectrometry-based approach to quantify the monomer–dimer equilibrium of the LPS transport protein LptH. We use this method to assess the potency and efficacy of an antimicrobial peptide and small molecule disruptors, obtaining new information on their structure–activity relationships. This approach led to the identification of quinoline-based hit compounds representing the basis for the development of novel LPS transport inhibitors.


 Please wait while we load your content...
Please wait while we load your content...