How electrophilic are 3-nitroindoles? Mechanistic investigations and application to a reagentless (4+2) cycloaddition†
Abstract
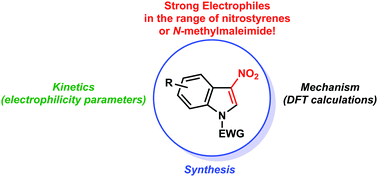
The electrophilicity of 4 different 3-nitroindole derivatives has been evaluated by Mayr’s linear free energy relationship (log k(20 °C) = sN(E + N)) and reveals unexpected values for aromatic compounds, in the nitrostyrene range. 3-Nitroindoles are sufficiently electrophilic to interact with a common diene namely the Danishefsky's diene at room temperature, in the absence of any activator, to furnish smoothly the dearomatized (4+2) cycloadducts in good yields.


 Please wait while we load your content...
Please wait while we load your content...