Synthesis of axially chiral biaryl thioglycosides through thiosugar-directed Pd-catalyzed asymmetric C–H activation†
Abstract
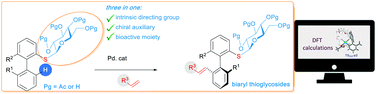
Herein we report for the first time that the thiosugar moiety can be used both as a directing group enabling the regioselective activation of a C–H bond of biaryl scaffolds and as a chiral source inducing axial chirality. Our approach enables the easy generation of complex thioglycoside atropoisomers, thus paving the way to new products of potential biological interest.
- This article is part of the themed collection: Functionalization of unreactive C-H bonds


 Please wait while we load your content...
Please wait while we load your content...