Bottom-up method for synthesis of layered lithium titanate nanoplates using ion precursor†
Abstract
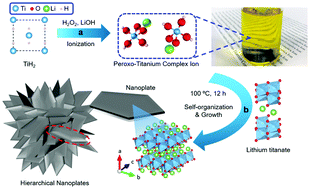
A facile bottom-up method for the synthesis of lithium titanate nanoplates using a peroxo titanium complex ion precursor is reported. Instead of employing complicated treatment with high alkali concentration, the self-organization reaction between lithium and titanium ions in the prepared ion precursor can enable the formation of layered lithium titanate crystals (Li2−xHxTi2O5, where x = 0.1 and 1.52 for as-synthesise and acid-treated samples, respectively) under low alkaline conditions. We demonstrate that layered lithium titanate crystals can be grown anisotropically into individual nanoplates. Our work presents an easy and useful platform for the production of titanate materials with various morphologies based on the interaction with ionic species.


 Please wait while we load your content...
Please wait while we load your content...