Metal-free enaminone C–N bond cyanation for the stereoselective synthesis of (E)- and (Z)-β-cyano enones†
Abstract
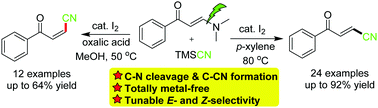
A highly practical method for C–CN bond formation by C–N bond cleavage on enaminones leading to the efficient synthesis of β-cyano enones is developed. The reactions take place efficiently to provide (E)-β-cyano enones with only a molecular iodine catalyst. In addition, the additional employment of oxalic acid enables the selective synthesis of (Z)-β-cyano enones.


 Please wait while we load your content...
Please wait while we load your content...