Kinetic resolution of N-aryl β-amino alcohols via asymmetric aminations of anilines†
Abstract
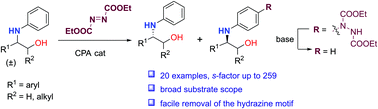
An efficient kinetic resolution of N-aryl β-amino alcohols has been developed via asymmetric para-aminations of anilines with azodicarboxylates enabled by chiral phosphoric acid catalysis. Broad substrate scope and high kinetic resolution performances were afforded with this method. Control experiments supported the critical roles of the NH and OH group in these reactions.


 Please wait while we load your content...
Please wait while we load your content...