Fast synthesis and redox switching of di- and tetra-substituted bisthioxanthylidene overcrowded alkenes†
Abstract
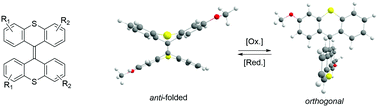
A rapid and efficient method for the synthesis of overcrowded alkenes using (trimethylsilyl)diazomethane provides a range of substituted bisthioxanthylidenes. We show large conformational redox switching from folded to orthogonal states, which tolerates many substitution patterns. The facile access to bisthioxanthylidene switches with the potential for further functionalization, in combination with the reliable redox chemistry, provides major opportunities for the design of electrochemically responsive systems.


 Please wait while we load your content...
Please wait while we load your content...