HFIP promoted thio(hetero)arylation of imidazoheterocycles under metal- and base-free conditions†
Abstract
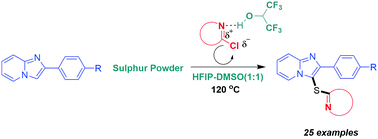
A new reaction methodology has been developed for HFIP promoted Thio(hetero)arylation of imidazoheterocycles under metal and base-free conditions. To the best of our knowledge, this is the first report that describes linking of imidazopyridines with electron deficient heteroarenes through a sulphur atom and also for the synthesis of most of these compounds. The reaction conditions are well tolerated by almost all kinds of 2-chloroheteroarenes and a wide range of imidazoheterocycles. The synthesized compounds can show significant biological properties.


 Please wait while we load your content...
Please wait while we load your content...