Acyl silane directed Cp*Rh(iii)-catalysed alkylation/annulation reactions†
Abstract
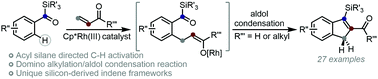
Studies into the Cp*Rh(III)-catalysed hydroarylation of alkenes with aryl acyl silanes led to the discovery of a new synthetic strategy to access unique silicon derived indene frameworks. Rather than protodemetalation of the metal enolate formed following insertion of an alkene into the aryl C–H bond, intramolecular aldol condensation of the acyl silane occurred to generate a series of 2-formyl- and 2-acetyl-3-silyl indenes. This represents only the second example of rhodium-catalysed C–H functionalisation employing acyl silanes as weakly coordinating directing groups and the intramolecular aldol condensation strategy was extended to access analogous silicon derived benzofurans.


 Please wait while we load your content...
Please wait while we load your content...