Copper-catalyzed aerobic double functionalization of benzylic C(sp3)–H bonds for the synthesis of 3-hydroxyisoindolinones†
Abstract
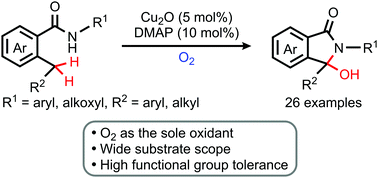
A copper-catalyzed aerobic 3-hydroxyisoindolinone synthesis was developed via the benzylic double C(sp3)–H functionalization of 2-alkylbenzamides. In this reaction, molecular oxygen was used as both an oxidant for C(sp3)–H functionalization and an oxygen source. Our method can be extended to diverse benzylic C(sp3)–H bonds and shows excellent functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...