Proton-accelerated Lewis acid catalysis for stereo- and regioselective isomerization of epoxides to allylic alcohols†
Abstract
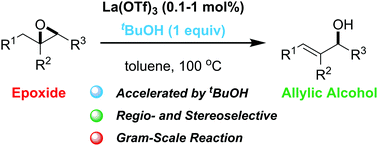
The isomerization of epoxides to allylic alcohols was developed via proton-accelerated Lewis acid catalysis. The addition of tBuOH as a proton source is the key to the efficient catalytic cycle. Trisubstituted epoxides, including enantioenriched derivatives, were selectively converted to secondary-allylic alcohols without loss of enantiopurity.


 Please wait while we load your content...
Please wait while we load your content...