Synthesis of α,β-unsaturated epoxy ketones utilizing a bifunctional sulfonium/phosphonium ylide†
Abstract
Herein, a new protocol for rapid synthesis of α,β-unsaturated epoxy ketones utilizing a bifunctional sulfonium/phosphonium ylide is described. This approach comprises two sequential chemoselective reactions between sulfonium and phosphonium ylides and two distinct aldehydes, which allows for the rapid construction of a variety of unsymmetric α,β-unsaturated epoxy ketones. This methodology allows the rapid construction of the core reactive functionality of a family of lipid peroxidation products, the epoxyketooctadecenoic acids, but can be further broadly utilized as a useful synthon for the synthesis of natural products, particularly those derived from oxidized fatty acids. Accordingly, a protocol utilizing this approach to synthesize the epoxyketooctadecenoic acid family of molecules is described.
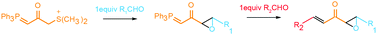


 Please wait while we load your content...
Please wait while we load your content...