Redox-active tetraaryldibenzoquinodimethanes
Abstract
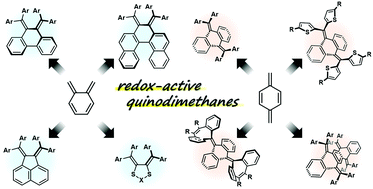
Quinodimethanes (QDs) are a class of non-aromatic π-conjugated compounds that are well-known to be interconvertible scaffolds in many response systems. While parent ortho- and para-QDs (o-QD and p-QD) can be easily converted to benzocyclobutenes or oligomers/polymers by the formation of C–C bonds at α-positions, the attachment of four phenyl groups to these reactive sites makes o-Ph4QD and p-Ph4QD long-lived. We have demonstrated that further dibenzo-annulation of such tetraaryl QD units also drastically increases their stability, and many tetraarylated dibenzoquinodimethane derivatives have been developed. This Feature Article shows our milestones in creating functional redox systems, where drastic changes in structure occur upon electron transfer (dynamic redox “dyrex” behaviour).


 Please wait while we load your content...
Please wait while we load your content...