Rapid incorporation of a difluoroacetate radical into para-quinone methides via radical 1,6-conjugate addition†
Abstract
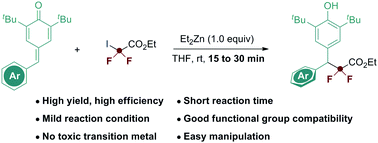
Presented herein is a newly designed strategy that rapidly introduces ethyl difluoroacetate radicals through a dialkylzincs induced radical 1,6-conjugate addition pathway. Besides achieving high yields and excellent functional group compatibility, this protocol allowed the incorporation of a gem-difluoromethylene motif to be accomplished within minutes.


 Please wait while we load your content...
Please wait while we load your content...