A modular olefination reaction between aldehydes and diborylsilylmethide lithium salts†
Abstract
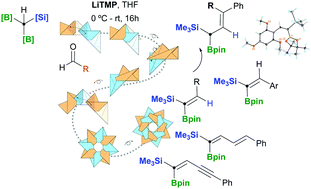
We describe the preparation of densely functionalised 1,1-silylborylated trisubstituted alkenes, via a boron-Wittig reaction, between LiC(Bpin)2(SiMe3) and aliphatic or aromatic aldehydes. The condensation of diborylsilylmethide lithium salts with α,β-unsaturated aldehydes provides a direct pathway to synthesize 1,1-silylborylated conjugated dienes and diynes.


 Please wait while we load your content...
Please wait while we load your content...