Iridium-catalysed branched-selective hydroacylation of 1,3-dienes with salicylaldehydes†
Abstract
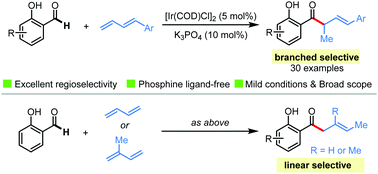
Herein, we report an iridium-catalyzed branched-selective hydroacylation of 1-aryl 1,3-dienes with salicylaldehydes under mild conditions with no need of phosphine ligands. With this protocol, a series of α-branched β,γ-unsaturated o-hydroxyacetophenones with biological potentials were synthesized in high efficiency with excellent regioselectivities. When simple 1,3-butadiene or isoprene instead of 1-aryl 1,3-dienes were used, exclusive linear-selective hydroacylation products were obtained.
- This article is part of the themed collection: 2021 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...