Synergistic Cu/Pd-catalyzed asymmetric allylation: a facile access to α-quaternary cysteine derivatives†
Abstract
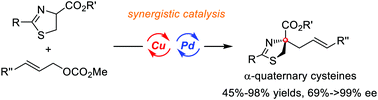
An efficient synthetic methodology to access biologically important and synthetically useful α-quaternary cysteine derivatives via asymmetric catalytic α-allylation of readily available 2-thiazoline-4-carboxylates was successfully developed through a synergistic Cu/Pd catalytic system. A wide array of α-quaternary cysteine derivatives were obtained in moderate to high yields with good to excellent enantioselectivities (45–98% yields and 69–>99% ee). Gram-scale asymmetric allylation was performed to obtain high yields maintaining the enantioselectivity. Moreover, some synthetic transformations to access chiral spirocyclic compounds proceeded smoothly, which exhibited the important utility of this methodology.


 Please wait while we load your content...
Please wait while we load your content...