Synthesis of acetic acid from CO2, CH3I and H2 using a water-soluble electron storage catalyst†
Abstract
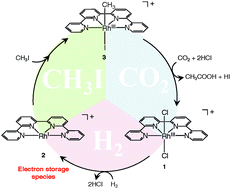
This paper reports a possible mechanism of acetic acid formation from CO2, CH3I and H2 in aqueous media and the central role played by a water-soluble Rh-based electron storage catalyst. In addition to water-solubility, we also report the crystal structures of two presumed intermediates. These findings together reveal (1) the advantage of water, not only as a green solvent, but also as a reactive Lewis base to extract H+ from H2, (2) the role of the metal (Rh) centre as a point for storing electrons from H2 and (3) the importance of an electron-withdrawing ligand (quaterpyridine, qpy) that supports electron storage.


 Please wait while we load your content...
Please wait while we load your content...