Chiral metal down to 4.2 K - a BDH-TTP radical-cation salt with spiroboronate anion B(2-chloromandelate)2−†
Abstract
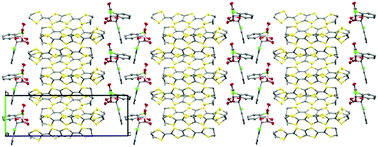
We report the first example of a chiral BDH-TTP radical-cation salt. Chirality is induced in the structure via the use of a chiral spiroboronate anion where three stereocentres are present, one on each chiral ligand and one on the boron centre. Despite starting from a labile racemic mixture of BS and BR enantiomers, only one enantiomer is present in the crystal lattice. The anions pack in a novel double anion layer which is the thickest anion layer found in a BDH-TTP salt. This material is chiral and shows metallic behaviour down to at least 4.2 K.


 Please wait while we load your content...
Please wait while we load your content...