Lewis base-free thiophosphonium ion: a cationic sulfur atom transfer reagent†
Abstract
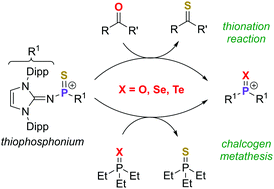
Phosphorus(V) sulfide and Lawesson's reagent are commonly used thionating reagents which are considered to operate after dissociation into highly reactive dithiophosphorane fragments. We report the synthesis and properties of a monomeric thiophosphonium ion [R2P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S]+. The highly electrophilic species reacts with carbonyls in oxo-for-sulfido exchange reactions at room temperature and undergoes phosphorus–chalcogen bond metathesis reactions with phosphine chalcogenides.
S]+. The highly electrophilic species reacts with carbonyls in oxo-for-sulfido exchange reactions at room temperature and undergoes phosphorus–chalcogen bond metathesis reactions with phosphine chalcogenides.
- This article is part of the themed collection: #RSCPoster Conference


 Please wait while we load your content...
Please wait while we load your content...