Recent developments in selective N-arylation of azoles
Abstract
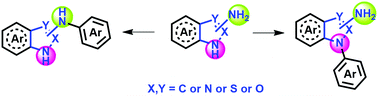
Transition-metal based carbon–heteroatom (C–X) bond formation has attracted the attention of synthetic chemists over the past few years because the resultant aryl/heteroaryl motifs are important substructures in many natural products, pharmaceuticals, etc. Several efficient protocols such as Buchwald–Hartwig amination, Ullmann coupling, Chan–Lam coupling and metal-free approaches have proved beneficial in C–X bond formation. Selective arylation of one hetero-centre over other centres without protection/deprotection thus allowing minimum synthetic manipulation has been achieved for several substrates using these protocols. Azoles are one such novel five-membered heterocyclic core with huge pharmaceutical applications. Though N-arylation on azole-bearing analogues has been extensively practised, selective N-arylation either on one N-centre or the exocyclic N-site of the azole ring in competition with other hetero-centres in the framework has been recently explored for azole-carrying systems. Thus, this review would focus on recent advances in chemo- and regio-selective N-arylation (either on one N-centre or the exocyclic N-site of the azole ring) on azole-containing frameworks.


 Please wait while we load your content...
Please wait while we load your content...