Selective cine-arylation of tert-cyclobutanols with indoles enabled by nickel catalysis†
Abstract
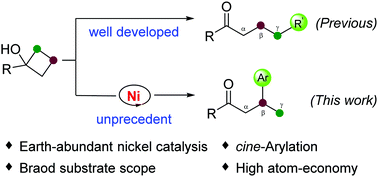
In previous literature, tert-cyclobutanols are widely studied for C–C bond activation exclusively leading to the formation of ordinary γ-substituted ketones. Herein, we first report a nickel-catalyzed cine-arylation of tert-cyclobutanols with indoles to access β-aryl ketones with an unusual site-selectivity at the C3-position of tert-cyclobutanols. The reaction features earth-abundant nickel catalysis, excellent regioselectivity, high atom-economy, and broad substrate scope.


 Please wait while we load your content...
Please wait while we load your content...