Iodide-promoted transformations of imidazopyridines into sulfur-bridged imidazopyridines or 1,2,4-thiadiazoles†
Abstract
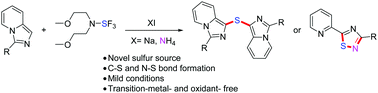
A NaI-promoted sequential double carbon–sulfur bond formation was developed to afford sulfur-bridged imidazopyridines, using Deoxofluor as the sulfur source and requiring only 15 min at room temperature. Using this process, imidazo[1,5-a]pyridines could also be transformed to 1,2,4-thiadiazoles in the presence of ammonium salt with the formation of both carbon–sulfur and nitrogen–sulfur bonds. This mechanistically unique method is distinguished by its wide substrate scope, lack of requirement for transition metals and mild conditions.


 Please wait while we load your content...
Please wait while we load your content...